FDA Requirements for Salt Export to the USA: A Complete Guide for Importers & Exporters
- Zayan Rauf

Key takeaways
- FDA facility registration is mandatory for all foreign salt manufacturers before exporting to the USA
- Prior Notice must be submitted 2-8 hours before shipment arrival depending on transportation mode
- Food-grade certification requires meeting 21 CFR standards and CGMP regulations
- Proper labeling must include product name, origin, net quantity, and manufacturer details
- Complete documentation includes commercial invoice, Bill of Lading, and Certificate of Analysis
- Pakistan exported 1,121+ shipments of Himalayan salt to USA between Nov 2023-Oct 2024
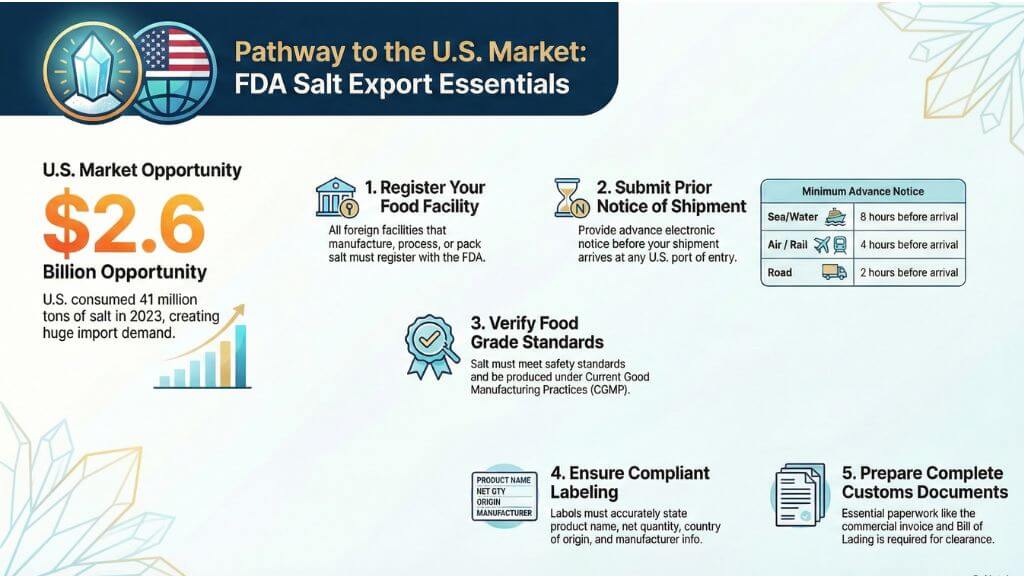
- USA salt market consumed 41 million tons worth $2.6 billion in 2023
- Working with experienced exporters like Sobaan Salts ensures compliance and smooth customs clearance
The United States is one of the world’s largest salt importers, with Pakistan emerging as a key supplier of premium Himalayan pink salt. For B2B exporters and importers, understanding FDA compliance is critical for smooth salt export to USA operations and avoiding costly customs delays.
This guide outlines every essential FDA requirement for exporting food-grade salt, helping international importers and B2B buyers navigate the complex regulatory landscape with confidence.
Understanding the USA Salt Import Market
The USA salt market is large and diverse. According to the U.S. Geological Survey, domestic salt consumption reached 41 million tons in 2023, valued at around $2.6 billion [1]. While the United States produces significant quantities domestically, it still depends heavily on imports to meet demand across various sectors.
Did You Know?
Pakistan exported over 1,121 shipments of Himalayan salt to the United States between November 2023 and October 2024, marking a 24% growth compared to the previous year [2]. The United States now accounts for 31% of Pakistan’s total salt exports.
Import data shows that Chile, Canada, Mexico, and Egypt have traditionally dominated USA salt imports. However, Pakistan’s Himalayan pink salt has grown remarkably in the American market. The pink salt demand in the USA has increased due to consumer preferences for natural, mineral-rich alternatives to regular table salt.
For Pakistani exporters, this creates a significant opportunity. The Pakistan to USA salt export corridor has become increasingly important, with competitive pink salt prices in international markets despite premium positioning.
Read More: How To Import Himalayan Salt From Pakistan
Core FDA Requirements for Salt Importers
1. Food Facility Registration (FFR)
The FDA requires all foreign food facilities that make, process, pack, or store food for U.S. consumption to register with the agency before exporting [3]. This rule applies to salt manufacturers and processors in Pakistan, including operations at facilities near the Khewra Salt Mine.
Key registration requirements include:
- Facilities must register online through the FDA’s Food Facility Registration Module (FFRM)
- Registration must be renewed every even-numbered year between October 1 and December 31
- A unique Food Facility Registration Number will be assigned upon successful registration
- Registration information must be updated within 60 days if any mandatory details change
Foreign salt exporters must name a U.S. Agent who serves as the domestic communication representative. This agent must physically live in the United States and have a place of business within the country. The U.S. Agent handles all communication between the FDA and the foreign facility.
The Sobaan Salt Company ensures full FDA facility registration for all manufacturing locations, providing clients with verified registration numbers that speed up the import process and build trust with U.S. buyers.
2. Prior Notice of Imported Food
Prior Notice is the most critical real-time requirement for salt export to USA operations. The Bioterrorism Act of 2002 requires that FDA receives advance electronic notification before food shipments arrive at U.S. ports [4].
Prior Notice submission timeline by transportation mode:
Transportation Mode | Minimum Advance Notice |
Road Transport | 2 hours before arrival |
Rail Transport | 4 hours before arrival |
Air Transport | 4 hours before arrival |
Water/Sea Transport | 8 hours before arrival |
Prior Notice can be submitted through two systems: FDA’s Prior Notice System Interface (PNSI) or U.S. Customs and Border Protection’s Automated Broker Interface (ABI). PNSI submissions can be made up to 15 calendar days before arrival. ABI submissions can be made up to 30 calendar days in advance.
Required information in Prior Notice includes:
- Identity and contact information of the submitter
- FDA Product Code for salt (typically 25010020 for rock salt/Himalayan salt)
- Complete manufacturer details including facility registration number
- Shipper, importer, and consignee information
- Estimated quantity from largest container to smallest package
- Country of origin and any countries where entry was previously refused
Once submitted, FDA reviews the Prior Notice and issues a Prior Notice Confirmation (PNC) Number. This confirmation must come with the shipment and be shown to Customs and Border Protection upon arrival. Not submitting proper Prior Notice can result in shipment refusal at the port of entry [5].
3. Food Grade Certification and Compliance
For salt products meant for human consumption, FDA compliance means meeting specific food safety and quality standards outlined in Title 21 of the Code of Federal Regulations (CFR).
Salt is generally recognized as safe (GRAS) when used according to good manufacturing practices. However, colored salts like Himalayan pink salt must meet additional requirements. The FDA’s guidance on colored sea salt says that any substances added to give color must be approved food-grade color additives [6].
Read More: Bulk Himalayan Salt Procurement: Navigating the Pakistani Market
Food-grade salt USA requirements include:
- Products must be produced under sanitary conditions that prevent adulteration
- Manufacturing processes must follow Current Good Manufacturing Practice (CGMP) regulations
- Salt must be free from contamination and harmful adulterants
- Quality control testing should verify mineral composition and purity
- Products should meet microbiological safety standards
Did You Know?
Himalayan pink salt gets its distinctive color from trace minerals like iron oxide. The FDA requires that these naturally occurring colorants do not make the salt unsafe or misbranded. According to mineral analysis, authentic Khewra salt typically contains 95-97% sodium chloride with 3-5% trace minerals [7].
4. Labeling Requirements
Proper labeling is essential for FDA compliance. All salt products entering the USA must have labels that provide accurate, complete, and truthful information to consumers.
Mandatory label elements for food-grade salt include:
- Product name and common or usual name (e.g., “Himalayan Pink Salt,” “Rock Salt”)
- Net quantity of contents in both metric and U.S. customary units
- Name and address of manufacturer, packer, or distributor
- Country of origin statement
- Ingredient declaration if any additives are present
- Nutrition Facts panel (if making nutrition claims or if package size requires it)
For Himalayan salt export to the USA, labels must clearly show the salt’s origin. Terms like “Himalayan Pink Salt” or “Pink Himalayan Salt” are acceptable. However, claims about health benefits must be carefully worded to avoid breaking FDA rules on disease claims.
The FDA’s food labeling guide gives detailed requirements under 21 CFR Part 101. Exporters should pay close attention to nutrient content claims and make sure sodium content is properly shown if a Nutrition Facts label is included.
Himalayan Sobaan Salt offers private labeling services with FDA-compliant label designs, ensuring that all regulatory requirements are met while maintaining brand identity for international clients.
5. Import Entry and Customs Documentation
Salt shipments must clear U.S. Customs and Border Protection (CBP) inspection before entering commercial distribution. The import entry process requires coordination between the importer, customs broker, FDA, and CBP.
Essential documentation includes:
- Commercial invoice with complete product description
- Packing list detailing shipment contents
- Bill of Lading or Airway Bill
- Prior Notice Confirmation Number
- FDA Food Facility Registration Number
- Harmonized Tariff Schedule (HTS) code (typically 2501.00 for salt)
- Certificate of Analysis (recommended but not always mandatory)
- Certificate of Origin
During the entry review process, FDA electronically screens all imported food products. If FDA needs more information or samples, importers may receive a “May Proceed” notice, a request for more information, or a “Refused Entry” notice.
Products that do not meet U.S. requirements may be refused admission. Refused products must be destroyed or exported within 90 days. Common reasons for refusal include missing Prior Notice, unregistered facilities, misbranded products, or suspected contamination.
Understanding Himalayan Pink Salt Export Regulations
Himalayan pink salt from Pakistan faces the same FDA requirements as other salt products but also benefits from specific market advantages. Premium exporters source directly from the Khewra Salt Mine region, one of the world’s oldest and purest salt deposits.
The pink salt demand in the USA continues to grow as consumers want natural, unprocessed alternatives. Research shows Americans are increasingly interested in specialty salts that offer trace minerals beyond standard table salt. While scientific studies show limited extra health benefits from trace minerals in pink salt, consumer perception drives market demand [8].
Quality Standards for Premium Export
Sobaan Salts Exporter maintains rigorous quality control throughout the supply chain:
- Direct sourcing from authenticated Khewra mine locations
- Multi-stage processing that preserves natural mineral content
- Laboratory testing for purity, mineral composition, and microbiological safety
- Moisture control and proper packaging to prevent contamination
- Full traceability from mine to port
For food-grade salt USA markets, quality assurance goes beyond meeting minimum FDA requirements. Premium suppliers invest in certifications like ISO 22000 (Food Safety Management) and HACCP (Hazard Analysis Critical Control Points) to show strong food safety systems.
Pakistan to USA Salt Export Logistics
Good logistics management is crucial for keeping product quality and meeting delivery times. Most Pakistan to USA salt export shipments go through major Pakistani ports like Karachi, then travel to U.S. ports including Los Angeles, New York/New Jersey, Houston, and Savannah.
Common FDA Compliance Challenges and Solutions
Challenge 1: Prior Notice Timing Errors
Many first-time exporters have trouble with Prior Notice submission timing. Submitting too early or too late can cause shipment delays.
Solution: Set up standard procedures with your freight forwarder or customs broker. Use automated systems that calculate the best Prior Notice submission times based on vessel schedules and transportation modes.
Challenge 2: Registration Number Confusion
Importers sometimes confuse facility registration numbers with other identification codes, leading to entry rejections.
Solution: Keep a master document with all registration numbers, company identifiers, and FDA product codes. Share this information with all supply chain partners including customs brokers, freight forwarders, and importers.
Challenge 3: Labeling Non-Compliance
Wrong or incomplete labels are a frequent cause of FDA detention or refusal.
Solution: Work with FDA compliance specialists to design labels before printing large quantities. Experienced exporters offer label review services and can recommend FDA-approved label designers familiar with food product requirements.
Challenge 4: Documentation Gaps
Missing or incomplete documents can trigger FDA holds or CBP examinations.
Solution: Create a complete shipping checklist that includes all required documents. Digital document management systems can help make sure nothing is missed. Keep digital and physical backups of all certificates and compliance documents.
Working with a Reliable Salt Supplier
Choosing the right export partner greatly affects FDA compliance success. Experienced Himalayan salt suppliers bring years of expertise in salt export to USA operations, with deep knowledge of both Pakistani regulations and American requirements.
Why Choose Professional Exporters for USA Trade
- Complete regulatory support: From facility registration to Prior Notice submission, established exporters handle all FDA compliance needs, allowing importers to focus on marketing and distribution.
- Quality assurance: Every batch undergoes rigorous testing for purity, mineral composition, and safety. Certificates of Analysis are provided with each shipment, giving importers confidence in product quality.
- Competitive pricing: Direct sourcing from mines removes middlemen, ensuring competitive pink salt prices in international markets without losing quality.
- Flexible packaging: Whether you need bulk containers for food manufacturers or retail-ready packages for consumer brands, professional suppliers offer customized packaging solutions that meet FDA labeling requirements.
- Reliable supply chain: Established relationships with freight forwarders and customs brokers in both Pakistan and the USA ensure on-time delivery and smooth customs clearance.
B2B Solutions for American Importers
Leading Pakistani exporters understand that B2B buyers have unique needs. Top suppliers work closely with:
- Food manufacturers seeking food-grade salt USA certified ingredients for production
- Retailers and distributors building private label Himalayan salt brands
- Wellness companies requiring spa-grade salt for therapeutic products
- Food service wholesalers supplying restaurants and commercial kitchens
Visit Sobaan Salts to explore a complete range of export-quality Himalayan salt products and request a quote for your specific requirements. Professional consultation is available on FDA compliance, logistics, and market entry strategies.
Staying Current with FDA Regulations
FDA rules change regularly. Recent developments include proposed changes to Prior Notice requirements for international mail shipments and ongoing efforts to reduce sodium intake across the U.S. food supply.
The FDA’s voluntary sodium reduction targets, published in 2021, encourage food manufacturers to slowly reduce sodium content. While these targets are voluntary, they may influence future rules and market preferences.
Conclusion
Successfully navigating FDA requirements for salt export to the USA requires attention to detail, complete documentation, and reliable partnerships. From food facility registration and Prior Notice submission to labeling compliance and quality assurance, each step needs careful execution. The growing Himalayan pink salt demand in the USA creates significant opportunities for Pakistani exporters who prioritize FDA compliance and quality standards.
For complete support with salt export to the USA, including FDA compliance help, quality certifications, competitive pricing, and logistics coordination, connect with Himalayan Salt Exporter today. Expert teams are ready to help navigate the complexities of international trade and build a strong presence in the American market.
References
[1]: U.S. Geological Survey. (2024). Mineral Commodity Summaries 2024: Salt. U.S. Department of the Interior. https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-salt.pdf
[2]: Volza. (2024). Himalayan Salt Exports from Pakistan to the United States. Trade Data Analysis (Nov 2023-Oct 2024). https://www.volza.com/p/himalayan-salt/export/export-from-pakistan/cod-united-states/
[3]: U.S. Food and Drug Administration. (2024). Registration and Listing: Food Facilities. FDA Basics for Industry. https://www.fda.gov/industry/fda-basics-industry/registration-and-listing
[4]: U.S. Food and Drug Administration. (2024). Prior Notice of Imported Foods. FDA Import Program. https://www.fda.gov/industry/fda-import-process/prior-notice-imported-foods
[5]: Code of Federal Regulations. (2024). 21 CFR Part 1 Subpart I – Requirements to Submit Prior Notice of Imported Food. Electronic Code of Federal Regulations. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-A/part-1/subpart-I
[6]: U.S. Food and Drug Administration. (2024). Guidance for Industry: Colored Sea Salt. Regulatory Information. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-colored-sea-salt
[7]: McGill University Office for Science and Society. (2019). Is Himalayan Pink Salt Better For You? Science & Society Analysis. https://www.mcgill.ca/oss/article/health-and-nutrition-quackery-you-asked/himalayan-pink-salt
[8]: Iodine Global Network. (2024). The Rising Popularity of Himalayan Pink Salt: Health Implications. Public Health Analysis. https://ign.org/latest/blog/the-rising-popularity-of-himalayan-pink-salt/
Share This Post
Article By

